Frontera to Present Innovative Gene Therapy Advances at the 2025 ARVO and ASGCT Annual Meetings
Frontera Therapeutics, a global innovator in recombinant AAV (rAAV)-based gene therapy research and manufacturing, continues to drive innovation across ophthalmology, hematology, and cardiovascular disease. In May 2025, Frontera will present its latest research achievements at the Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting and the 28th Annual Meeting of the American Society of Gene & Cell Therapy (ASGCT).
2025 ARVO Annual Meeting
Date: May 4–8, 2025
Location: Salt Lake City, Utah, USA
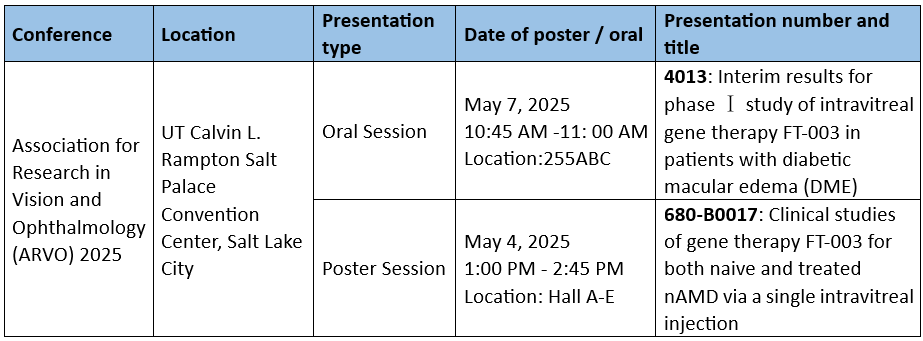
At ARVO, Frontera will deliver an oral presentation on the Phase I clinical trial progress of FT-003, an investigational AAV gene therapy for Diabetic Macular Edema (DME). FT-003 is the first AAV gene therapy targeting studied in China and represents a critical step toward understanding the risk-benefit profile of gene therapies in chronic eye diseases.
Additionally, a poster presentation will highlight preliminary efficacy and safety data for FT-003 in patients with Neovascular Age-Related Macular Degeneration (nAMD), including both treatment-naïve and previously treated patients.
28th ASGCT Annual Meeting
Date: May 13–17, 2025
Location: New Orleans, Louisiana, USA
Frontera will present one oral presentation and four posters at the 2025 ASGCT Annual Meeting:
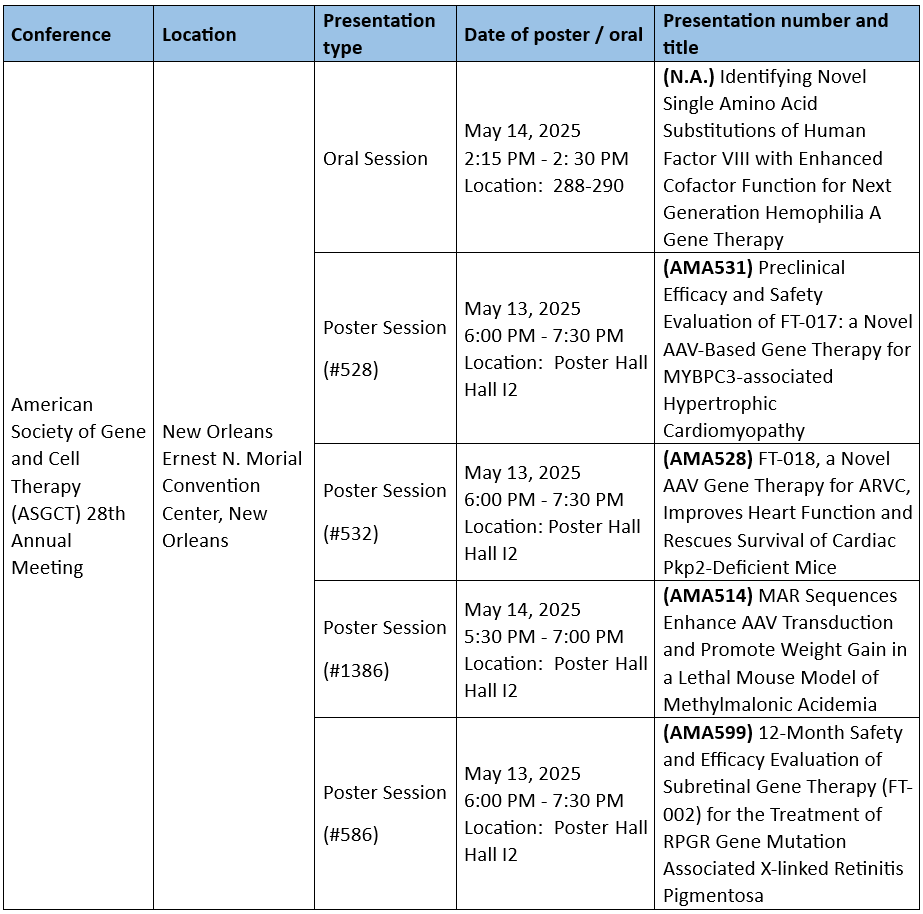
• The oral presentation will unveil the breakthrough development of a next-generation gene therapy for Hemophilia A, utilizing advanced site-directed mutagenesis technology.
• Two poster presentations will highlight preclinical research progress of FT-017 and FT-018, novel gene therapies for cardiovascular indications.
• Another poster will share 12-month follow-up clinical data for FT-002, an AAV-based gene therapy for X-linked Retinitis Pigmentosa (XLRP), demonstrating its long-term safety and potential clinical benefit.
Global R&D and Manufacturing Excellence
Frontera has built a robust global platform, with R&D operations in Boston, USA, and clinical and GMP manufacturing facilities in Shanghai and Suzhou, China. Its proprietary insect-cell-based rAAV production system has reached 500 L scale and is scalable to 2000 L, offering flexibility and efficiency. Frontera has also developed innovative purification technologies to enhance safety and reduce manufacturing costs—reflecting its commitment to delivering high-quality, affordable gene therapies worldwide.
Vision for the Future
“Our gene therapy programs are demonstrating First-in-Class and Best-in-Class potential in clinical development,” said Dr. Xinyan Li, CEO of Frontera. “Presenting at ARVO and ASGCT allows us to share our breakthroughs, collaborate with global leaders, and accelerate the delivery of transformative therapies to patients worldwide.”



 沪公网安备31011502401398号
沪公网安备31011502401398号